Textbooks often describe enzymes are highly efficient, have high rate of reaction, and much superior to that of a chemical catalyst. But how fast is it possible for an enzyme-catalyzed reaction to proceed? Is there any limit to the rate acceleration achievable by enzymes? The answer is yes, there is a certain upper limit to the rate acceleration by an enzyme and very few enzymes have achieved it. This special class of enzyme is known as “Diffusion Limited Enzyme.
Diffusion Limited Enzyme
The rate is limited by the rate at which the substrate can diffuse onto its active site and product diffusion away into the solution. Every encounter between the enzyme and the substrate leads to the product. But do all the enzymes fall into the diffusion-limit zone or do all the enzymes are kinetically superior? To answer these questions lets first understand the evolution of different kinetic parameters of an enzymatic reaction.
It is believed that natural selection and evolution shape the present day’s three-dimensional structure and function of enzymes. Similarly, natural selection also plays a central role in the evolution of different enzyme kinetic parameters (kcat, KM). Traditionally, the enzyme activity is defined by the parameters kcat/KM, though other alternatives are also proposed.
Natural Selection of Enzyme’s Kinetic Parammnetrs
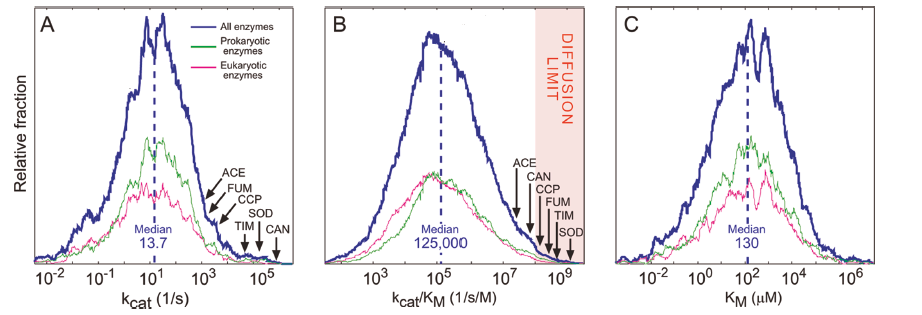
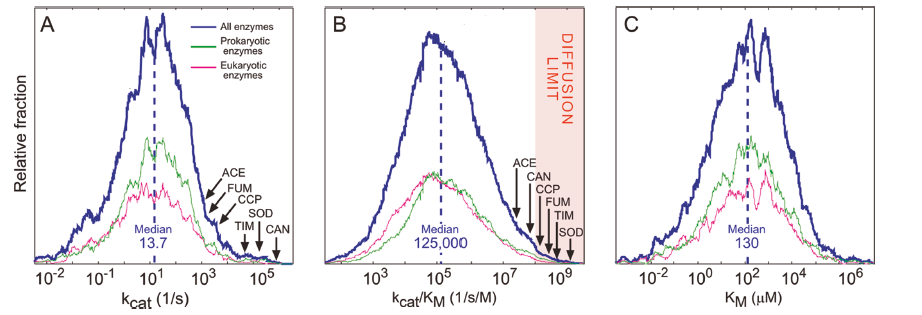
To study the impact of evolution on the kinetic parameters, Milo and co-workers mined the Brenda and KEGG database to analyze these parameters for several thousands of enzymes1. The Brenda database provides two key metrics of enzyme performance: kcat, a first-order rate constant related to the turnover number, and kcat/KM, a second-order rate constant referred to as the catalytic efficiency. The KEGG database differentiates between the natural (native) substrates with the non-native substrates. The results obtained after analyzing the datasets uncover the general trends and deduce some interesting conclusions.
From the above plots, it is observed that the kinetic parameters of most of the enzymes fall into the average region which means the enzymes are far less catalytically efficient than the textbook example or diffusion-limited enzymes. That is why most of the enzymes are called “average enzymes”.
Average Enzymes
The “average enzymes” exhibits a kcat/KM value nearly four orders of magnitude lower than the diffusion limit. This gives an important observation that evolution is not pushing enzymatic rates towards the diffusion limit. The probable reason for the average nature of most of the enzymes is physiological constraints. The enzymatic turnover rate is driven by the need to maintain an appropriate metabolic flux, which is sufficient conversion of substrate to product per unit time, thus maximizing substrate. The evolution drives enzymes toward good enough rather than perfect to maintain the cellular functions.
References
- Bar-Even, A.; Noor, E.; Savir, Y.; Liebermeister, W.; Davidi, D.; Tawfik, D. S.; Milo, R. The Moderately Efficient Enzyme: Evolutionary and Physicochemical Trends Shaping Enzyme Parameters. Biochemistry 2011 , 50 (21), 4402–4410. https://doi.org/10.1021/bi2002289.