Biocatalysis and cofactor
Biocatalysis has been extensively used in the chemical and pharmaceutical industries for the manufacture of products ranging from specialty to commodity chemicals. Enzymes are employed in the commercial synthesis of chiral intermediates/products used in the synthesis of new drugs. However, many of these enzymatic redox reactions require one or more cofactors that are consumed during reaction.
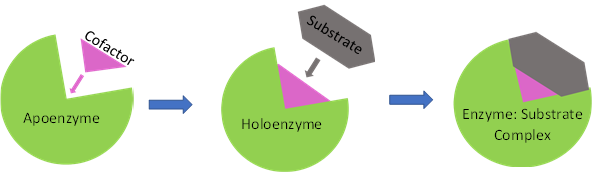
Cofactors are non-protein organic molecules or metal ions that assist enzymes in catalysing chemical/biological reactions. Cofactors perform several functions such as regulation of substrate binding to the enzyme, facilitating oxidation-reduction reactions and by stabilizing charges on the enzyme. Enzymes exist as apoenzymes which upon binding to cofactors undergo conformational changes to become holoenzyme that are effective biocatalysts (Fig. 1). For instance, enzymatic reductions require a cofactor (or coenzyme) as the hydride donor, notably NADH and its phosphorylated form, NADPH. Owing to the expensive cost of cofactors, a stoichiometric supply is not economically viable. An effective and robust system of cofactor regeneration is the need of the hour for large scale applications in the industry.
Fig 1. Enzyme - cofactor interactions aiding substrate binding
Cofactor recycling
Cofactor recycling is a process which makes use of an external agent that is not directly involved in the reaction of interest but ensures the continuous supply of cofactors for efficient biocatalysis. Development of a cofactor regeneration system must contemplate factors such as activity, selectivity, process sustainability, by-product generation and compatibility with production enzymes. The enzymatic reaction must not experience mutual deactivation between the regeneration and production systems.<br><br>
Methods of cofactor recycling
In general, methods for cofactor regeneration can be sub-divided into six categories as follows:
1. Enzymatic regeneration
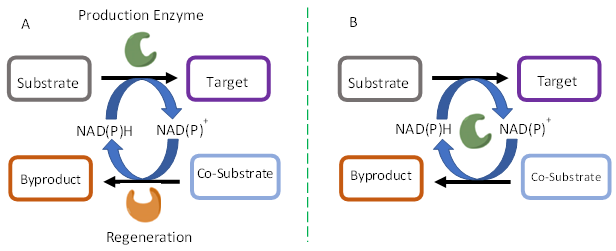
Enzymatic regeneration approaches are highly compatible with the target bioconversions due to their overlapping reaction conditions including room temperature process in an aqueous medium at near neutral pH. Two common strategies employed are (A) Coupled enzyme wherein a regeneration enzyme and an associated co-substrate are used, (B) Coupled substrate wherein the same enzyme performs both substrate conversion and cofactor regeneration. Strategy ‘A’ might be much more effective in cofactor regeneration as the load on the enzyme is lesser but it will incur the cost of two enzymes for a single bioconversion reaction. On the other hand, strategy ‘B’ might be economically viable but the enzyme loading and the associated cost will be higher since two reactions are performed by the same enzyme.
Fig 2. Enzyme Regeneration Methods: A. Coupled Enzyme B. Coupled Substrate
2. Chemical Regeneration
This method involves exploitation of high redox potential of salts or cofactor analogues to achieve recycling of cofactors. Common reducing agents such as sodium dithionite, sodium borohydride and 1,4-dihydropyridines have been used historically as hydride donors for recycling. But chemical method has several issues including the large amount of the regeneration agent and high concentration of by-products generated that deactivate the production enzymes.
3. Electrochemical Regeneration
Electrochemical methods for cofactor regeneration by controlling the electrode potentials connected to the system. For example, NAD(P)+ generated by enzymatic process is reduced on the electrode surface to give protonated NAD(P)H. However, the radicals obtained in the enzymatic step can combine to form inactive dimers as side products leading to failure in regeneration.
4. Photocatalytic Regeneration
Photocatalytic regeneration is based on natural photosynthesis, that employs light-harvesting systems to generate electrons and electron transport chains through electron carrier proteins that results in cofactor regeneration. Development of high-performance photocatalysts involves mainly organic photosensitizers, inorganic semiconductors and light absorbing polymers such as carbon nitride.
5. Heterogeneous Catalytic Regeneration
This method can be utilized in enzymatic reactions wherein hydrogen gas is one of the reactants/substrates. Heterogeneous catalysts such as Pt, Pd, Rh, Ru, Ni, Au, and Ag are well established in activating hydrogen and promoting reduction reactions with minimal downstream separations. The protons released by the chemical catalysts can be both consumed in bioconversion and cofactor regeneration, thereby attaining 100% atom efficiency. But this method faces disadvantages such as poor cost effectiveness and lower activity for NADP+ based reactions.
Enzyme based regeneration methods
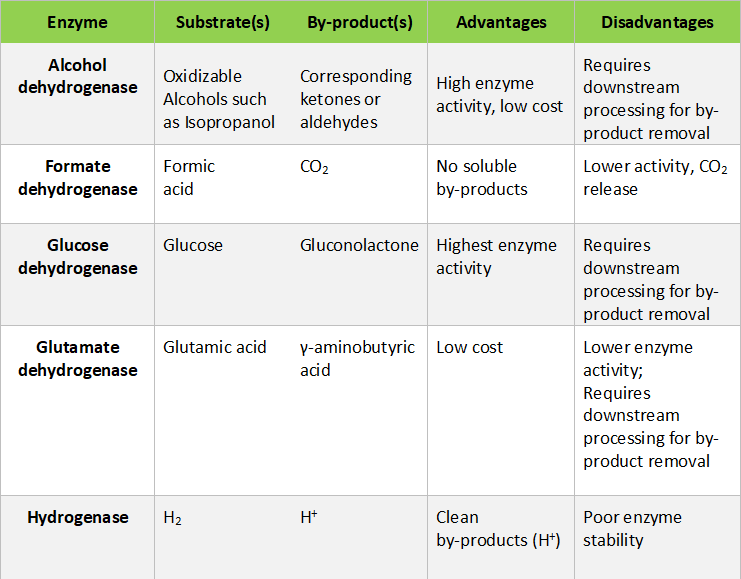
Among the different methods used for cofactor regeneration, enzymatic regeneration is the most applicable for large scale industrial operations. The auxiliary enzymes used for regeneration provide many advantages such as high specific activity, mild operational conditions, easier recovery and recycle (upon immobilization) and cleaner by-products that simplify downstream processing of required products. A comparative study of the different enzymes that can be explored for cofactor regeneration is provided in the below table:
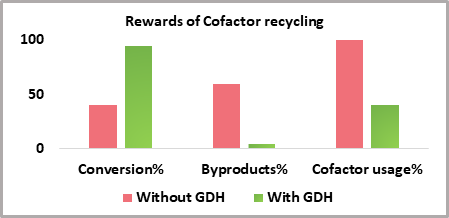
We at Quantumzyme™, have successfully employed enzyme-based cofactor regeneration. We have made use of a coupled enzyme - glucose dehydrogenase (GDH) for recycling the expensive NADPH cofactor. The GDH enzyme has been effectively used for the ketoreductase based bioconversion of a novel ketone substrate to its corresponding chiral alcohol. The chiral product is extensively used in the pharmaceutical industry for API production. The cofactor recycling by GDH has presented us with immense advantages as depicted below:
Quantumzyme™, being an enzyme engineering and biotransformation company, are relentlessly exercising innovations to reduce the industrial bioconversion economics, progress towards green chemistry and continue to support the scientific community through our research.