
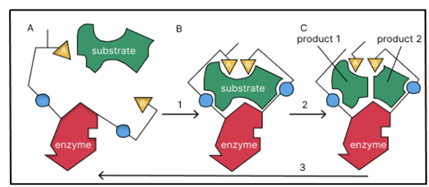
Enzymes are large biomolecules, typically proteins, that catalyze biochemical reactions by binding to specific substrates and converting them into products. The ability of enzymes to recognize and bind to their substrates is critical for their catalytic activity. Enzyme and substrate binding is like a lock and key mechanism: each enzyme has a specific set of substrate(s) that it can bind to. Depending on the chemical reaction, the enzyme can either convert a single substrate into product(s) or combine two substrates and form the product. But how does an enzyme recognize the substrate?
The process of substrate recognition by enzymes involves a series of specific interactions between the enzyme and the substrate. As the first step, the substrate and enzyme must come in close proximity to each other for the reaction to occur. Enzymes have a specific binding site, referred as active site, where the substrate binds. When the substrate molecule approaches the enzyme, it undergoes the process of recognition, and it is drawn towards the active site and oriented in a specific way. This can happen through a variety of mechanisms, depending on the specific context. For example, in some cases, if the enzyme is soluble in the aqueous environment surrounding the substrate, the substrate reaches the active site by diffusion or process of mixing. In cases of enzymes embedded in a membrane, such as ion channels or receptors, or the enzyme wants to act on substrate in another cell, the substrate crosses the membrane or extracellular fluid through diffusion, active transport, bulk flow, receptor-mediated transport, etc. and reaches the active site.
In some cases, interactions between the enzyme and the substrate occur outside of the active site, often at a distance from the active site. These interactions can help to guide the substrate towards the active site and enhance the specificity of the enzyme for the substrate. For example, some enzymes have "accessory" or "regulatory" domains that are located far from the active site and are involved in substrate recognition. These domains can recognize specific features of the substrate or other molecules that are involved in the reaction, such as co-factors or other enzymes in a metabolic pathway. The accessory domains can then guide the substrate towards the active site and help to position it correctly for catalysis. In addition, some enzymes have "allosteric" sites that can bind to specific molecules and cause a conformational change in the enzyme that alters its ability to bind to the substrate at the active site. This can change the efficiency of substrate recognition by the enzyme.
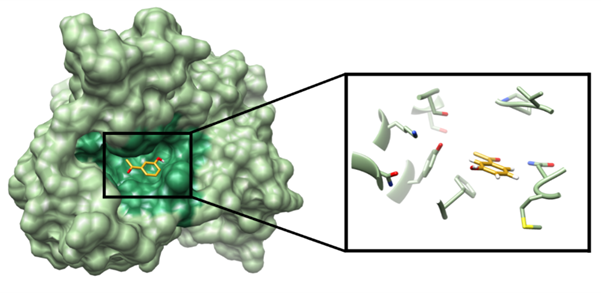
The shape and size of the substrate must complement that of the active site so that the enzyme can bind to the substrate with high specificity and selectivity. The active site is composed of a unique combination of amino acid residues of different sizes, acid-base properties, hydrophilicity, hydrophobicity, and charge. These residues are arranged in a particular sequence and structure that creates a distinct chemical environment in the active site. The distribution of charges on the enzyme and substrate can play a critical role in substrate recognition. Electrostatic interactions between oppositely charged residues on the enzyme and substrate can facilitate the binding process. Additionally, hydrogen bonding and van der Waals interactions can also contribute to substrate recognition.
Once the substrate is in the correct position within the active site, it sometimes undergoes a conformational change to form an enzyme-substrate complex. The shape of the enzyme changes because of the substrate attaching to the active site, improving the fit between the enzyme and substrate, which is also known as induced-fit mechanism. This process is facilitated by various chemical interactions such as hydrogen bonding, electrostatic interactions, and van der Waals forces.
An enzyme must develop very exact interactions with the substrate(s) it is intended to bind to. The shape and charge/lack of charge of a protein must precisely fit with its target, for it to form very specific interactions with its substrate(s). Therefore, an enzyme will only successfully "act on" molecules that correspond to the enzyme's surfaces, just as a specific key only operates in a lock that it fits well with.
The rate of enzyme–catalyzed reactions also depend on the concentration of enzyme and substrate. As the concentration of substrate or enzyme increases, the rate of the reaction also increases until a saturation point is reached and increasing the substrate concentration further does not increase the reaction rate.
Temperature and pH fluctuations can affect an enzyme's structure and form, since enzymes are sensitive to these changes. This, in turn, may have an impact on the enzyme's ability to bind to its substrate. Each enzyme has an optimal pH and temperature range in which it is most active. Deviations from this range can cause the enzyme to denature, lose its activity, or alter its specificity.
In conclusion, enzymes use a range of mechanisms to recognize their substrates, including the lock and key model, induced fit mechanism, flexibility, etc. The specificity of enzymes in recognizing their substrates is critical for maintaining the accuracy and efficiency of biochemical reactions in living organisms. As we continue to explore the fascinating world of enzyme-substrate recognition, we will undoubtedly uncover new and exciting insights into the biochemical processes that drive life.
Video link: How Enzymes Work (from PDB-101)