
Introduction
Enzymes are biocatalysts that play a pivotal role in the bioprocess industry. In recent years, they have replaced chemical catalysts because of their enantioselectivity, high substrate specificity, efficiency and green chemistry. Even though enzymes offer several advantages, one of the biggest challenges faced by biotechnologists is the difficulty to reuse/recycle enzymes economically. This problem was solved by the discovery of enzyme immobilization technology. Enzyme immobilization is a technique in which enzymes are confined to a carrier matrix, thereby improving stability, catalytic activity, and recovery rate. As shown in table 1, enzyme immobilisation offers several advantages over free enzyme; therefore, among the commercial users, enzyme immobilization has proved to be efficient and cost-effective.
Aspect | Free Enzyme Biocatalysis | Immobilized Enzyme Biocatalysis |
Enzyme stability | Free enzymes are less stable and can be easily denatured or inactivated | Immobilization enhances enzyme stability, provides protection against denaturation |
Reusability | Not reusable, as they may mix with the reaction mixture and cannot be collected in a physical form | Immobilized enzymes are generally easily separated from reaction mixture by simple filtration process, allowing multiple cycles of usage and increased cost-effectiveness |
Catalytic efficiency | Lower catalytic efficiency due to shorter active life in the reaction environment | Exhibit higher catalytic efficiency as they maintain activity over longer duration, leading to increased productivity |
Ease of separation | Separation of enzymes from the reaction mixture involves destruction of the enzymes and may require additional downstream processing steps | Can be easily separated from the reaction mixture, simplifying downstream processing and reducing final product purification costs |
Environmental impact | The process may generate more waste and require higher enzyme concentrations, contributing to environmental concerns | Immobilized enzyme systems can be more environmentally friendly, as they allow recyclability, lower enzyme concentrations and reduced waste generation |
Scale-Up Challenges | Scaling up of free enzyme processes can be challenging due to the need for larger volumes and increased difficulty in separation | Immobilized enzyme processes are often more amenable to scale-up, with easier separation and reduced processing challenges at larger scales |
Table 1: Comparison of free enzyme and immobilised enzyme
Selection of Immobilization Carrier
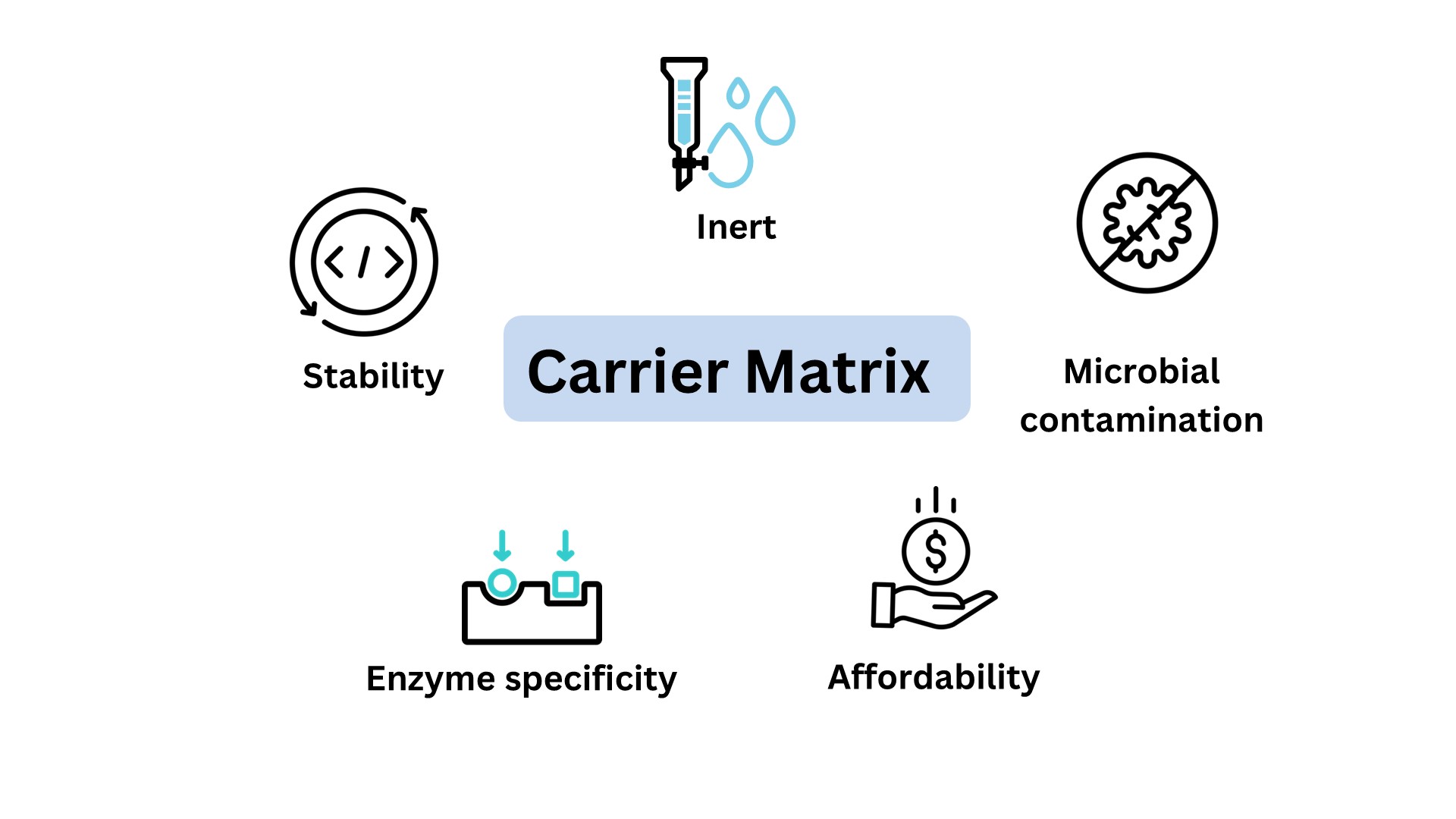
The efficiency of enzyme immobilization depends on carrier matrix properties and the immobilization method. Both these factors contribute to the enzyme stability thus affecting the substrate selectivity and the kinetics. An ideal matrix must possess the following characteristics: affordability, inertness, physical strength, stability, nonspecific adsorption, microbial contamination and the ability to enhance enzyme specificity and activity while lowering product inhibition. Choosing the right carrier matrix is a crucial task since it influences biocatalysis. A wide range of matrix materials are available and are, in general, classified as organic and inorganic materials.
Silica is the preferred inorganic material for enzyme immobilisation because of its sorption capacity, mechanical, chemical and thermal resistance. Moreover, the retainment of catalytic activity after repeated use makes it an ideal choice among users. For example, lipase immobilised on silica has shown higher activity (96%) compared to free enzymes. Inorganic oxides like titanium, aluminium and zirconium oxides have been used in the immobilisation of lipase, urease and α-amylase. Unlike silica and inorganic oxides, which possess only hydroxyl functional group, mineral materials have a wide range like -OH, -COOH, -SH and NH2 on the surface which helps in interaction with different classes of enzymes. In general, bentonite, halloysite, kaolinite and hydroxyapatite crystals are used.
In addition to inorganic materials, organic materials like chitosan, alginate, collagen, cellulose, starch, pectin and Sepharose, which are natural polymers, are preferred for their biocompatibility and availability. Chitosan a poly-amino-saccharide is of particular interest because of its malleability and ability to form multiple positive charges in acidic solutions. This feature helps in the interaction with negatively charged molecules. Another versatile polymer is calcium alginate, derived from seaweeds, with superior gel-forming and stabilising properties. In contrast, synthetic polymers are chemically derived, and the composition of polymers can be changed based on the enzyme’s properties. The type of functional group (carbonyl, carboxyl, hydroxyl, epoxy, amine and diol) on the monomer can be modified to facilitate effective binding of the enzyme. For example, immobilisation of tyrosinase via amine and carbonyl groups on polyamide 66.
Figure 1: Properties of carrier matrix
Methods of Immobilization
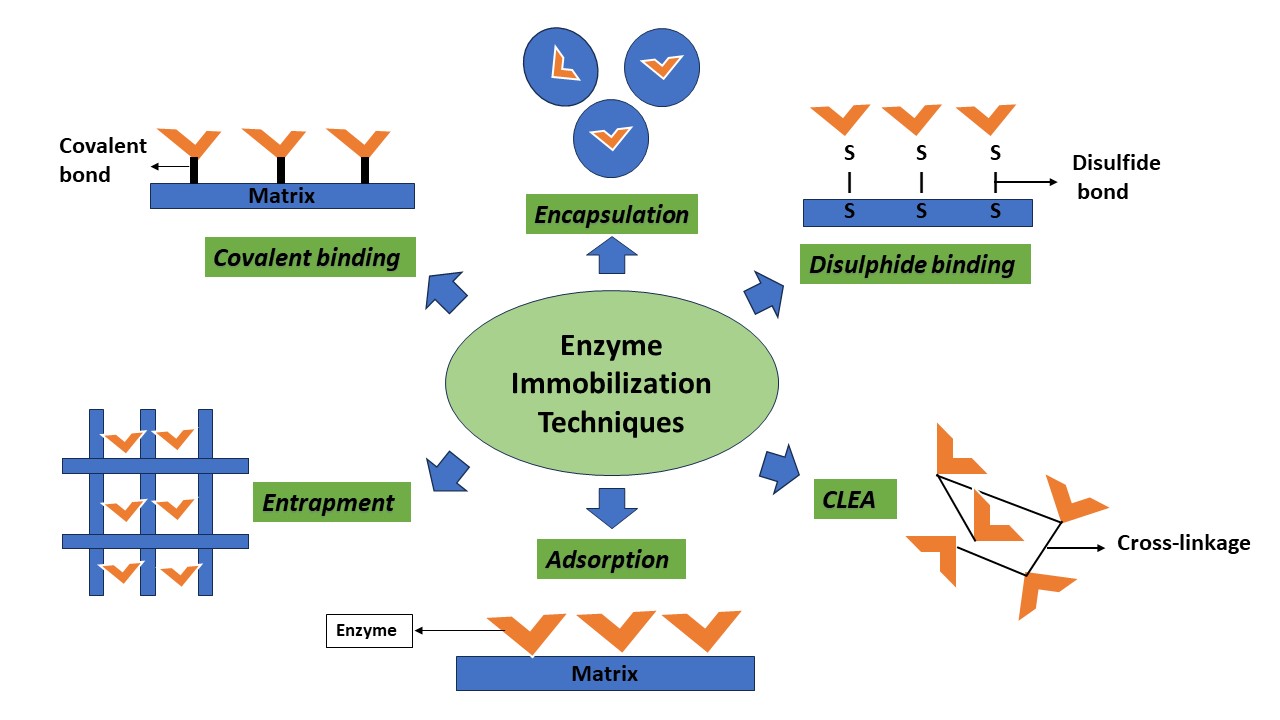
Once an ideal matrix is chosen, the enzyme is immobilised by reversible or irreversible methods based on the nature of chemical bond formed between the matrix and enzyme.
- Reversible methods
- Adsorption
Adsorption is a reversible process in which ionic or physical forces such as hydrogen bonds, van der Waals forces, or hydrophobic interactions are used to bind the enzyme to the matrix. Enzymes that have been adsorbed are protected against aggregation, proteolysis, and contact with hydrophobic surfaces. Not all enzymes can be immobilised by adsorption; immobilisation is only possible if the enzyme has affinity for the functional group present on the matrix. If absent, then surface modifiers are added to form connections between the enzyme and matrix. Few of the commonly used support materials are alumina, silica gel, calcium phosphate, starch, and carboxymethyl cellulose. Even though adsorption is a widely used technique, it has few disadvantages: loss of enzyme from the support after repeated use and non-specific binding of reaction components.
- Disulphide binding
In this approach, the free thiol group in the enzyme forms a stable covalent bond with the matrix. The matrix should be thiol-reactive i.e., it should have reactive disulphides or disulphide oxides. The reversibility of the bonds produced between the activated solid phase and the thiol-enzyme is an advantage as it allows the bound protein to be released with an excess of a low-molecular-weight thiol, such as dithiothreitol [DTT]. Enzymes for which the thiol bond is essential for structural stability cannot be used as it might lead to inactivation.
- Irreversible methods
- Covalent binding
A stable and strong linkage is created between the matrix and the enzyme during covalent binding. Typically, the activation steps of immobilization technique produce electrophilic groups on the matrix, which interact with the potent nucleophiles on the proteins during the coupling stage. The side chains of cysteine, lysine, glutamic acid, and aspartic acid are most frequently employed in this process. The major problem faced in this method is the requirement of additional modifications in certain enzymes such that the side chains are exposed for covalent binding to occur, but this can lead to denaturation and loss of activity.
- Entrapment and Encapsulation
In entrapment, an enzyme is trapped inside a polymeric gel (matrix) composed of either carrageenan, cellulose, agar, gelatine or collagen. The enzyme-polymer matrix permits only the products and substrates to pass through. It is necessary to modify the polymer’s pore size to prevent the loss of enzyme via diffusion. Another kind of entrapment technique is encapsulation, which involves packaging the enzyme in a semi-permeable membrane capsule such as cellulose or nitrate-based membrane. Since the enzyme is trapped in a membrane, enzyme leakage and denaturation, due to reaction with polymer, is prevented. But if the membrane thickness is high, a mass transfer opposition is created wherein the substrate is unable to diffuse and reach the enzyme’s active site.
- Cross-linked enzyme aggregation
Cross-linked enzyme aggregation method (CLEA) consists of two steps: the first step is to precipitate the enzyme with the help of precipitants like ammonium sulphate or tert-butanol. Subsequently, the enzymes undergo a cross-linking process in which a covalent bond is established between the functional group of the cross-linking agent and the free amino group, primarily lysine, resulting in the formation of an insoluble catalyst. A commonly used crosslinking agent is glutaraldehyde. Cross linked enzymes are resistant to organic solvents and high temperature. They are generally preferred in industries because of their stability, recyclability and volumetric efficiency. However, it is a very expensive method.
Figure 2: Enzyme immobilisation techniques. Orange colour represents enzyme, and blue colour represents immobilization carrier matrix
Immobilised enzymes in Industry
In an industrial process, the immobilised enzyme is coupled with bioreactors to perform large-scale biocatalysis. The choice of reactor depends on the productivity scale, down-stream processing and cost. Additionally, the kinetics of the reaction and the chemical and physical properties of the carrier matrix also play a major role. Few of the commonly used reactors for biocatalysis are discussed below.
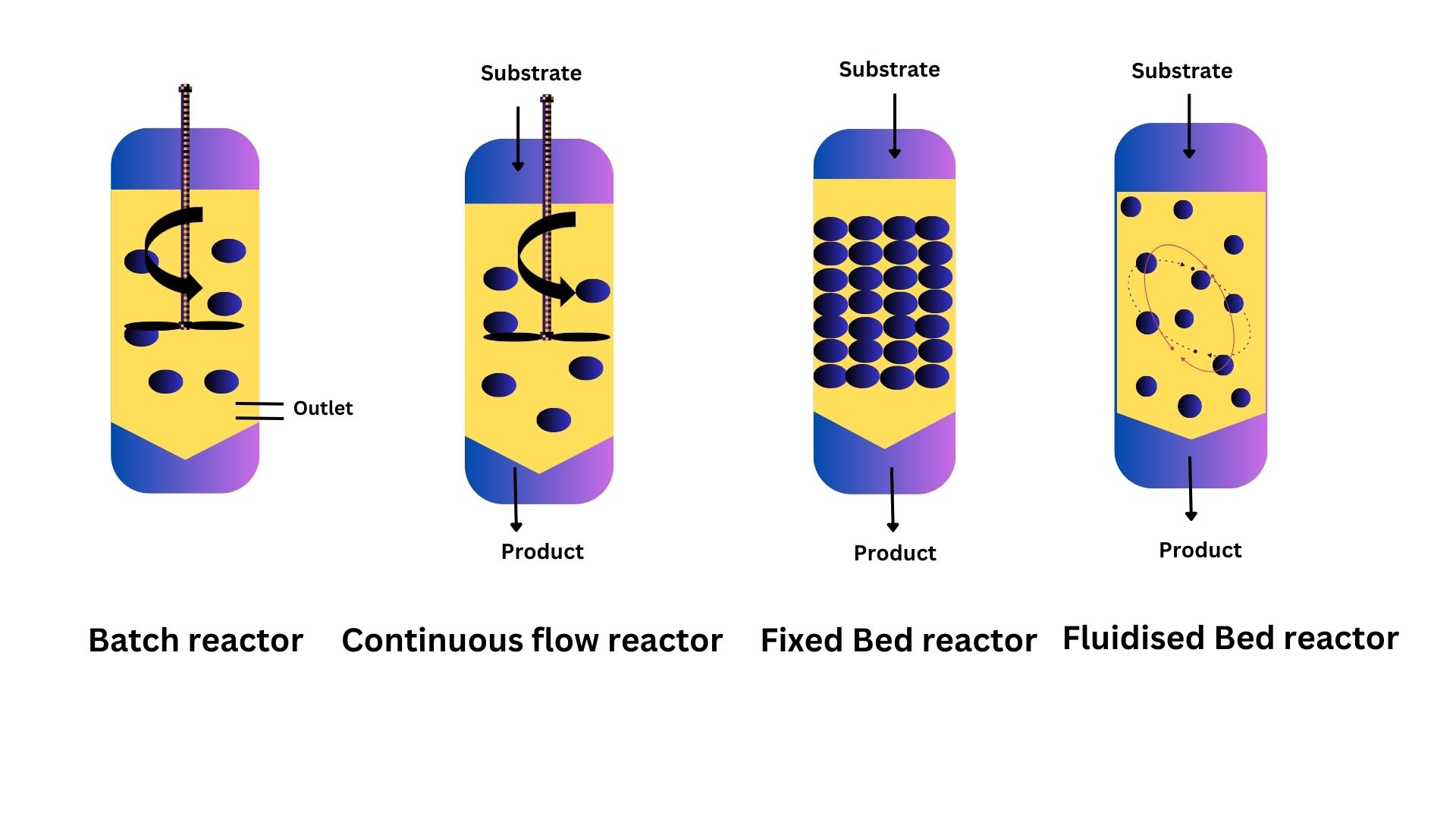
Batch reactors are generally used in the pharmaceutical industry for generating small volumes of product. In these types of reactors, the tank contains a stirrer which helps in aeration and mixing of the immobilised enzyme and substrate. Moreover, the immobilised enzyme and substrate are added simultaneously such that the residence time is similar within the reactor. In certain batch reactors like the fed-batch, there is addition of the substrate or immobilised enzyme at different timepoints. The major problem with batch reactors is the cost for operation and batch to batch variations. This is because the reactors must be emptied and refilled regularly leading to changes in reaction conditions making it difficult for scale-up.
Continuous flow reactors (CFR) are preferred for achieving higher productivity from a fixed amount of enzyme. A continuous stream of substrate is passed through a bed of immobilised enzymes, resulting in short substrate residence time. This constant reaction condition helps in the production of a purer and reproducible product.
A fluidized bed reactor is the method of choice for viscous substrates and products. The immobilised enzyme is suspended in a substrate stream, or in combination with inert gas, thereby mixing the enzyme and substrate to obtain a homogenous system. Fluidized bed reactor offers several advantages like ease in operation parameters, high mass and heat transfer rates. However, the unpredictability of fluidisation patterns and back mixing are drawbacks. The problem of back mixing can be overcome by using a fixed bed reactor in which the stream of substrate flows parallel to the reactor axis. The reactor is packed with immobilised enzymes thus creating a plug. The substrate stream passes through the bed of immobilised enzymes. Fixed bed reactors are mainly used by the food, chemical and pharmaceutical industries to increase productivity.
Figure 3: Different types of reactors used for biocatalysis. The blue dots represent immobilised enzymes, and the yellow is reaction medium
How to choose the right reactor for your application?
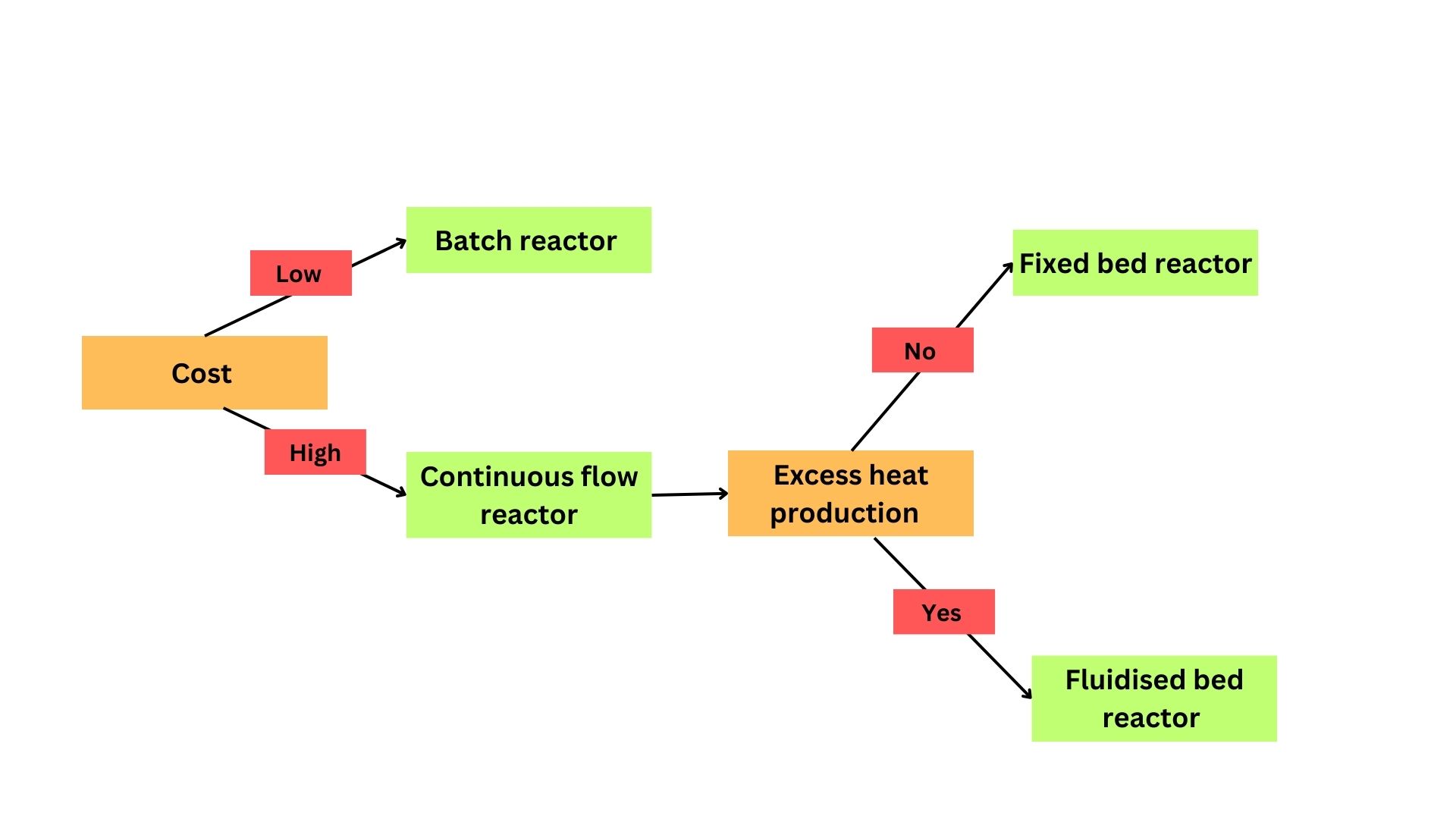
Cost is the crucial factor that drives the choice of reactor. Before one begins the process of scaling-up, economic evaluation is necessary. The following factors are prudent in cost evaluation: native enzyme, immobilisation matrix, downstream processing, reactors, disposal of immobilised enzyme and carrier regeneration. Out of these factors, choosing the correct reactor for scale-up can reduce the cost by huge margins.
Continuous flow reactors are favoured over batch reactors due to their scalability and rapid reaction rate. Continuous flow reactors have a shorter downtime and are 90% more efficient than batch reactors, which have a higher downtime due to the need to clean, heat, and cool the reactor before each new batch commences. Yet, due to their affordability and versatility, batch process reactors continue to rule the chemical production industry. Fluidised bed reactors are suitable for exothermic reactions, specifically if the heat liberated can damage the adsorbent. However, equipment and construction costs are higher for fluidised bed when compared to fixed bed reactors.
Figure 4: Flowchart depicting the major factors that determine the choice of reactor
Industrial Application: A Case Study
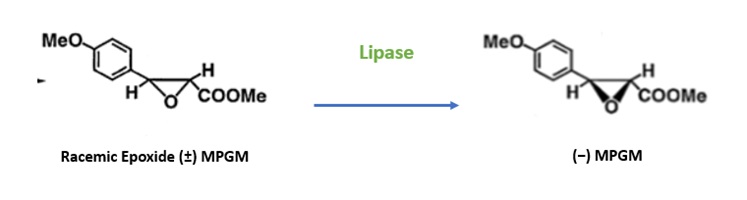
Diltiazem, a calcium channel blocker, is used in the treatment of cardiovascular diseases. It is an asymmetric carbon with four possible isomers of which only the (+) –(2S,3S)-isomer shows potent pharmaceutical properties. To synthesise diltiazem, the conventional chemical synthesis route involves nine steps which results in the generation of large quantity of wastes and high cost. This exhausting process can be shortened using hydrolase family enzymes.
Hydrolases can convert the racemic mixture of Methyl 3(4-methoxyphenyl) glycidate (MPGM) to enantiopure (-) MPGM, which is a key intermediate of diltiazem.
Figure 5: Enzyme catalysed enantioselective hydrolysis of racemic (+/-) MPGM to (-) MPGM
We at Quantumzyme™, have successfully optimised and employed immobilised hydrolase enzyme for the conversion of racemic MPGM to enantiopure (-) MPGM. As shown in Figure 5, immobilised enzyme showed higher enantioselective hydrolysis of racemic MPGM compared to free enzyme.
Aspect | Immobilised enzyme | Free enzyme |
Cost | Comparatively High | Low |
Conversion | >90% achieved | ~80% achieved |
Recyclability | Yes | No |
Catayltic Efficiency | High | Moderate |
Impurities | <5% | 30-40% |
Table 2: Comparison of enzymatic and free enzyme resolution of racemic MPGM
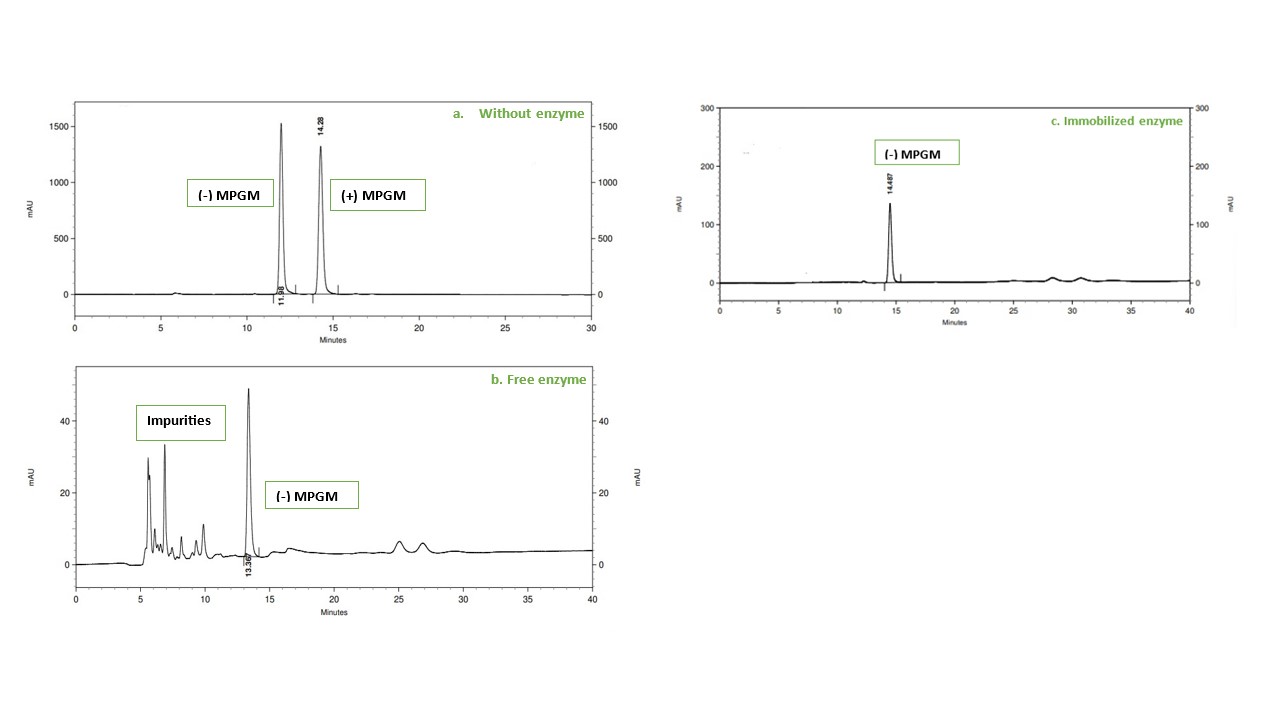
Figure 6: Chiral HPLC analysis of enzymatic hydrolysis of racemic MPGM
As observed in the HPLC results, both free enzyme and immobilized enzymes show complete racemate hydrolysis. But free enzyme biocatalysis produces vast impurities whereas immobilised enzymes produce almost negligible amount of impurities.
As an enzyme engineering and biotransformation company, Quantumzyme™ is committed to continuous innovation with the goal of reducing the economic impact of industrial biocatalysis, promoting the principles of green chemistry, and continuously contributing to the scientific community through our research endeavours.
References:
- Datta, Sumitra, et al. “Enzyme Immobilization: An Overview on Techniques and Support Materials.” 3 Biotech, 3 (1), 2013, 1– 9, https://doi.org/10.1007/s13205-012-0071-7.
- Maghraby, Yasmin, et al. “Enzyme Immobilization Technologies and Industrial Applications.” ACS Omega, 8 (6), 2023, 5184 – 96, https://doi.org/10.1021/acsomega.2c07560.
- Basso, Alessandra, and Simona Serban. “Industrial Applications of Immobilized Enzymes—A Review.” Molecular Catalysis, 479, 2019, 110607, https://doi.org/10.1016/j.mcat.2019.110607
- Malmiri, Hoda Jafarizadeh, et al. “Potential Applications of Chitosan Nanoparticles as Novel Support in Enzyme Immobilization.” American Journal of Biochemistry and Biotechnology, 8 (4), 2012, 203–19, https://doi.org/10.3844/ajbbsp.2012.203.219.