
Biocatalysis is an exciting field that taps into the power of natural catalysts, like enzymes, to drive chemical reactions in the production of active pharmaceutical ingredients (APIs). Industries mostly use enzymes in the form of total cell lysates. But here’s the catch: working with liquid lysates can be a bit of a rollercoaster ride. They often come with stability issues and contamination risks that can complicate the entire process. Enter lyophilization—a game-changing technique that enhances enzyme stability and minimizes microbial contamination, paving the way for more efficient and reliable production in pharmaceuticals.
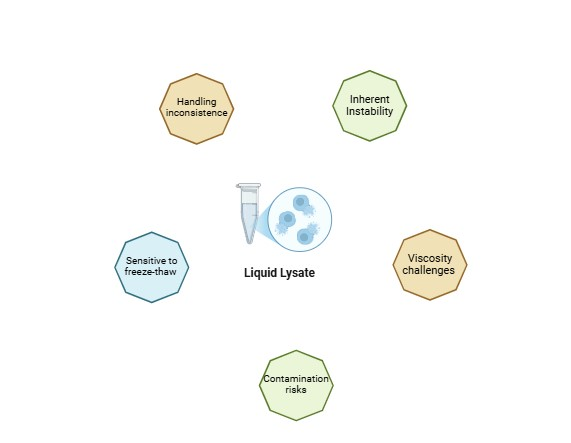
Challenges in working with liquid lysate:
Let’s discuss liquid lysates for a moment. Liquid lysates are commonly used in research and diagnostics, but they bring a host of challenges that can hinder their effectiveness. Working with liquid lysates is a constant battle against their instability. Here are some of the challenges that are encountered:
- Inherent Instability: These lysates can degrade over time, which is challenging when we aim for reliable and consistent results. Enzyme activity is lost due to the inherent proteolytic activity.
- Handling Inconsistencies: Even small variations in handling—like pipetting errors—can lead to uneven enzyme distribution, especially in high-throughput settings where reproducibility is crucial.
- Viscosity Issues: Some lysates are quite viscous, making accurate dispensing a challenge and potentially impacting assay performance.
- Contamination Risks: Liquid lysates are susceptible to contamination if not handled under strict aseptic conditions, requiring meticulous protocols that can be time-consuming.
- Sensitivity to Freeze-Thaw Cycles: Repeated freeze-thaw cycles can degrade enzyme activity, affecting data quality.
Lyophilization really shines here as the ultimate solution by stabilizing enzymes and enhancing their shelf life while reducing the risks associated with liquid handling.
Figure 1. Problems working with liquid lysate
Liquid vs. Lyophilized Enzymes: A Comparative Insight
Liquid enzymes and lyophilized enzymes serve distinct roles in biopharmaceutical applications, primarily influenced by their physical states and stability profiles. Liquid enzymes, typically maintained in a solution, offer immediate activity and are ideal for applications requiring rapid enzymatic reactions. However, they can be sensitive to temperature and pH, which means their storage must be done meticulously to preserve activity.
On the other hand, lyophilized enzymes are like the reliable friend that you can count on for the long haul. The lysates are lyophilized into a powder, which offers enhanced stability, shelf-life, reduced moisture content, minimal microbial growth and easier logistics. Lyophilized enzymes upon rehydration, spring back to life and are ready to work their magic.
Additionally, lyophilization allows for easier handling and dosage formulation, facilitating integration into various manufacturing processes. Ultimately, the choice between liquid and lyophilized enzymes hinges on specific application requirements, including stability, activity, and ease of use, underscoring the importance of understanding their biochemical properties.
Table 1. A comprehensive comparison of Lyophilized and Liquid enzymes
Feature | Liquid enzymes | Lyophilized enzymes |
State | Aqueous solution | Powder form |
Stability | Less stable; Sensitive to temperature and pH | More stable: Extended shelf life |
Storage conditions | Requires refrigeration or controlled conditions | Room temperature |
Activation | Immediate active upon use | Requires rehydration before use |
Shelf life | Shorter shelf life (Weeks) | Longer shelf life (Months) |
Handling | Requires careful handling to avoid contamination | Easier to transport and handle |
Application | Suitable for rapid reactions | Ideal for long term storage and transport |
Cost | Lower initial cost | Comparatively higher |
Enzyme activity | High activity in optimal conditions | High activity post optimisation of lyophilisation |
The Art of Lyophilization
Now, let’s dive into lyophilization process itself. Lyophilization, or freeze drying, is a critical process in the biological and pharmaceutical industries, enabling the conversion of unstable liquid formulations into stable, solid products. This technique is particularly valuable for preserving enzymes, which are often sensitive to environmental changes and require careful handling to maintain their stability.
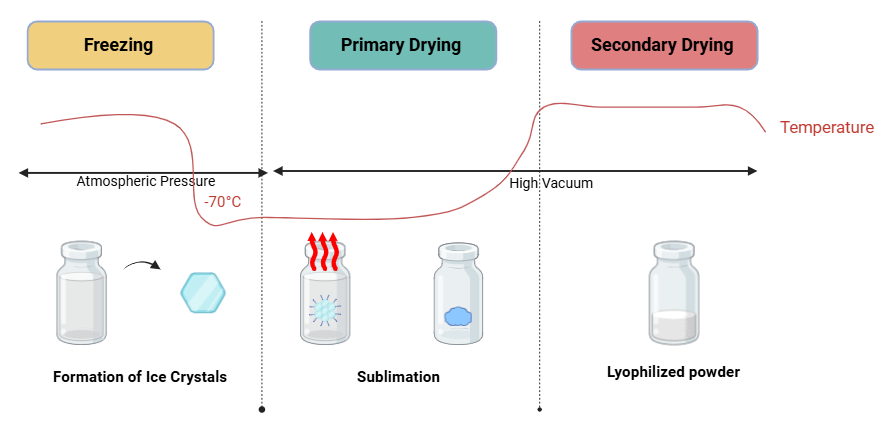
Lyophilization involves three main stages, and their optimization is the most critical process:
- Freezing: Freeze-Concentration Dynamics
This is where the magic begins. During freezing, the liquid formulation is cooled, leading to ice formation and the remaining soluble particles become more concentrated. It’s crucial to get this stage right to ensure enzyme stability. This freeze-concentration process can create high-stress conditions for enzymes, as they are typically trapped in an ice prison. To get it right, we need to carefully control the freezing rates and temperatures that minimizes the destabilizing effects and ensures a uniform distribution of ice crystals, which can help preserve enzyme activity.
- Primary Drying (Sublimation) - Managing Ice Removal
Here, the ice is sublimed into directly vapor, removing bulk of the water. This is where the real balancing act happens to ensure efficient removal of ice without causing thermal damage to the enzymes. Temperature of the shelf is carefully regulated to compensate for the heat lost during sublimation, while the vapor pressure in the chamber is maintained below that of ice vapor pressure.
- Secondary Drying - Reducing Bound Water
This phase removes any remaining bound moisture, which is vital for stability. Optimizing this step can make all the difference in maintaining enzyme efficacy.
Figure 2. Stages of lyophilization
Factors Affecting Enzyme Stability During Lyophilization
1. Cryoprotectants and Stabilizers
When it comes to preserving enzyme activity during lyophilization, cryoprotectants and stabilizers are absolute heroes. These compounds help protect enzymes from the stresses associated with freezing and drying. Commonly used cryoprotectants including sugars like trehalose and sucrose form a protective glassy matrix around enzymes which stabilizes the enzyme structure.
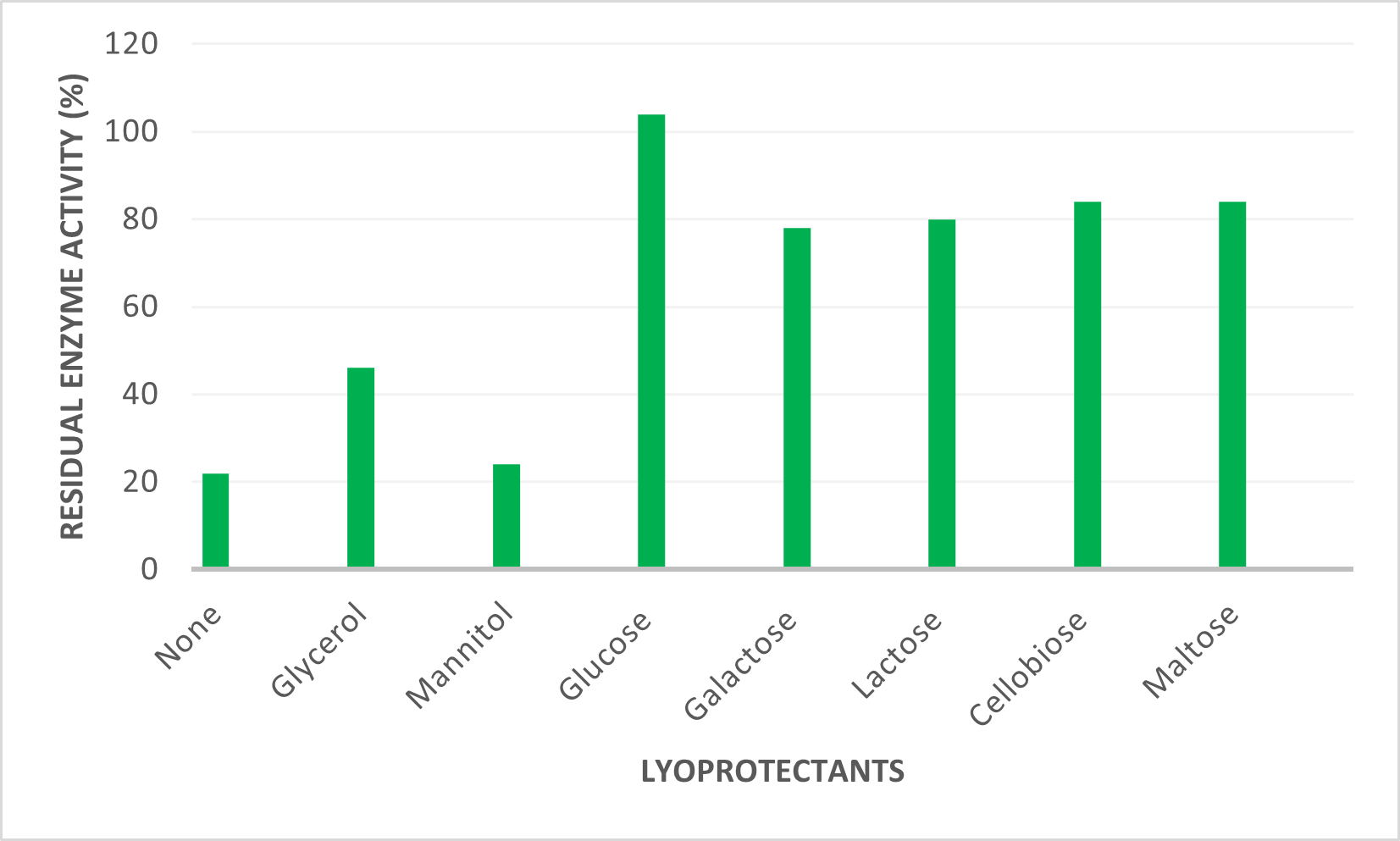
Workings of the SUGAR army in protecting enzymes
The water replacement hypothesis suggests that sugar molecules form hydrogen bonds with dried proteins, maintaining their structure and protecting them during drying and storage. Research shows that low-crystallinity carbohydrates preserve enzyme activity better than high-crystallinity sugars. After lyophilization, enzymes retain only 20-30% activity without lyoprotectants. Among the lyoprotectants, sugar alcohols such as mannitol is less effective while sugars like glucose, lactose, cellobiose, maltose and lactulose preserve over 80% of enzyme activity. Galactose and raffinose are comparatively less efficient sugars, but their residual activity is notably higher than that of sugar alcohols.
Figure 3. Case study of residual enzyme activity after lyophilisation with different lyoprotectants.
2. Lyophilization Conditions
To produce high-quality lyophilized products that meet industrial demands, we need to optimize key factors in the freeze-drying process. The conditions we choose for lyophilization—like freezing rates, shelf temperatures, and drying times—play a crucial role in enzyme stability. Each enzyme has its quirks, and fine-tuning the lyophilization parameters accordingly is essential. Adjusting the freezing rates, shelf temperatures and drying duration would help maximizing the stability. Regular monitoring and adjustments during the lyophilization cycle can help address any issues that arise.
2.1 Lyophilization cycle optimization:
To provide top-quality lyophilized products that meet industrial demands, the freeze-drying process must be optimized for key lyophilization cycle factors. While the primary drying parameters affect water loss, drying rates, and storage stability, the secondary drying parameters influence product stability, moisture content and microbial contamination.
3. Post-Lyophilization: Handling and Storage
Once the lyophilization process is complete, the handling and storage of the lyophilized product are crucial for maintaining its quality. The product should be kept sealed in its container and protected from exposure to moisture. Despite having less than 1% moisture content, lyophilized products can readily absorb moisture from the environment, which can deteriorate their quality and performance. Proper storage conditions—such as low humidity and controlled temperatures (based on enzymes) are essential to preserve the performance, shelf life, and reconstitution qualities of the lyophilized enzymes. It’s all about creating the right environment to ensure those enzymes stay effective!
Why lyophilization is so energy – intensive?
It is quite fascinating that significant energy is needed during lyophilization to turn ice into vapor at extremely low temperatures. It takes a whopping 2800 Joules of energy to convert just 1 gram of ice into water vapor. That is literally six times more energy than what is needed for the straightforward evaporation process that turns liquid into gas!
What about regulatory compliances?
Let’s briefly discuss the implications for regulatory compliance. When it comes to market approval and maintaining public trust, adhering to the guidelines set by regulatory bodies like the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) is crucial. Compliance in lyophilization isn’t just a one-time checklist; it’s a complex, ongoing process that starts from the formulation stage and continues all the way through post-market monitoring.
Maintaining detailed and precise records of the entire lyophilization process is one of the essential requirements for regulatory compliance. To optimize the stability of lyophilized enzymes, we can employ some strategic approaches. For instance, adjusting lyophilization parameters, selecting the right cryoprotectants, and ensuring proper storage after processing can significantly enhance the stability and functionality of enzymes in their lyophilized form. These strategies not only boost the efficiency of the lyophilization process but also play a vital role in the long-term success of biological and pharmaceutical products in the market. It’s all about creating a solid foundation for products that people can trust!
Conclusion
In conclusion, the journey from liquid lysates to lyophilization represents a significant advancement in biocatalysis, enhancing the stability and reliability of enzymes used in pharmaceutical production. By tackling the challenges of liquid lysates—like instability and contamination—lyophilization emerges as an absolute game-changer. The careful optimization of each stage of the freeze-drying process, from freezing to secondary drying, along with the strategic use of cryoprotectants and proper storage conditions, ensures that enzymes maintain their efficacy and functionality. As we continue to refine these techniques, we not only improve the efficiency of the lyophilization process but also contribute to the long-term success of biological and pharmaceutical products in the market. Embracing these advancements ensures that we are not just preserving enzymes but also enhancing the future of biocatalysis in the healthcare industry.
References:
- Suzuki, T., Imamura, K., Yamamoto, K., Satoh, T., & Okazaki, M. (1997). Thermal stabilization of freeze-dried enzymes by sugars. In Journal of Chemical Engineering of Japan (Vol. 30, Issue 4, pp. 609–613). https://doi.org/10.1252/jcej.30.609
- Pardeshi, S. R., Deshmukh, N. S., Telange, D. R., Nangare, S. N., Sonar, Y. Y., Lakade, S. H., Harde, M. T., Pardeshi, C. V., Gholap, A., Deshmukh, P. K., & More, M. P. (2023). Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: a state-of-the-art review. Future Journal of Pharmaceutical Sciences, 9(1). https://doi.org/10.1186/s43094-023-00551-8
- Molnar, A., Lakat, T., Hosszu, A., Szebeni, B., Balogh, A., Orfi, L., Szabo, A. J., Fekete, A., & Hodrea, J. (2021). Lyophilization and homogenization of biological samples improves reproducibility and reduces standard deviation in molecular biology techniques. Amino Acids, 53(6), 917–928. https://doi.org/10.1007/s00726-021-02994-w