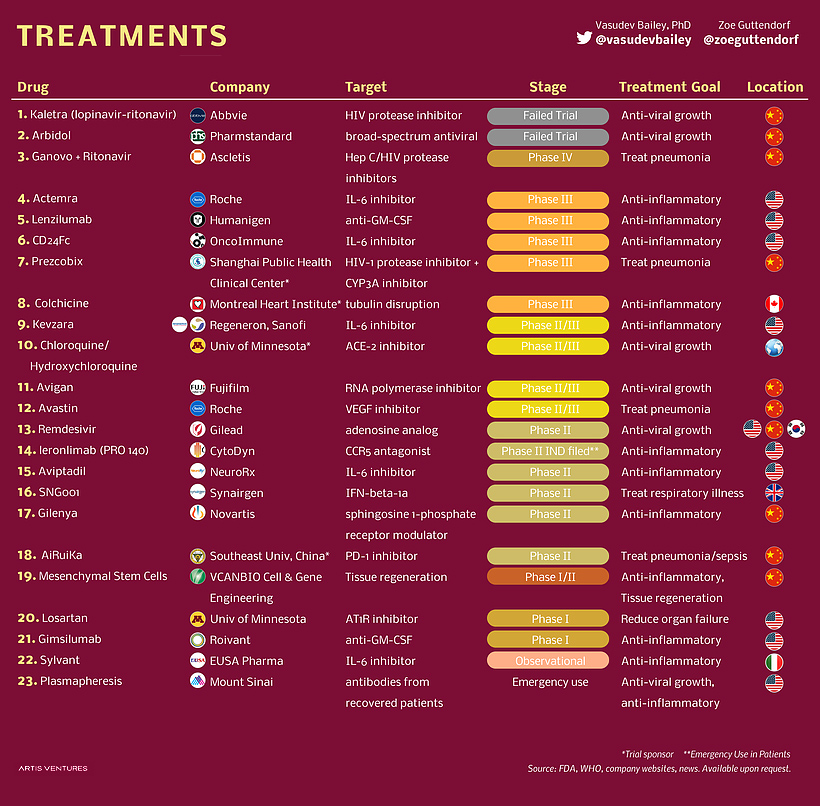
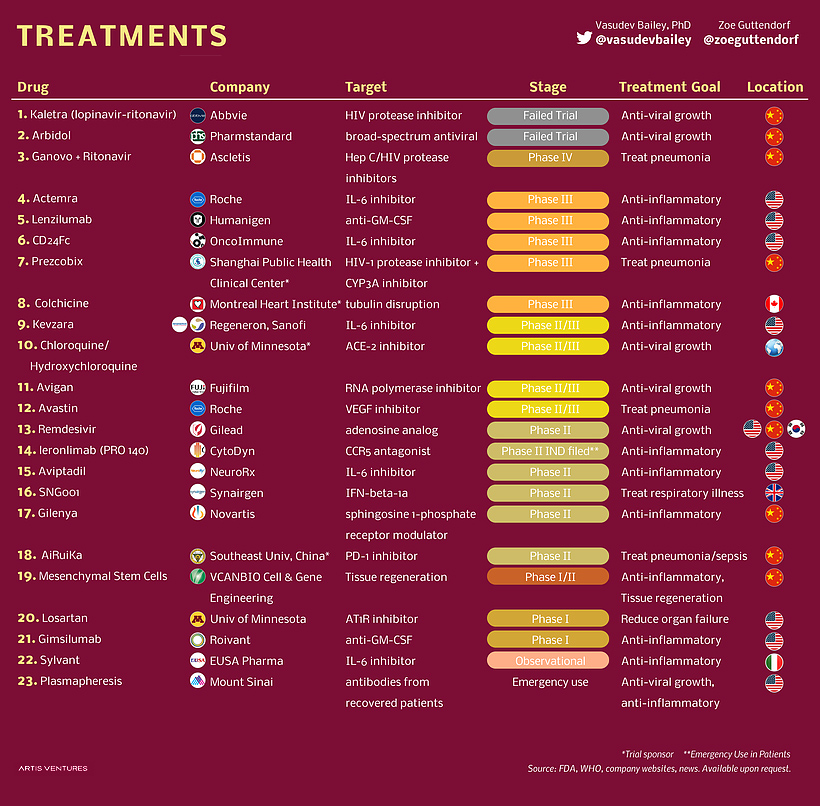
The pandemic of Coronavirus has become an everyday topic of discussion by affecting more than 8 million people globally. As India entered the top 5 list of Corona affected countries in the world, it has increased the urgency for a potential drug that can cure or at least reduce the effect of the virus. With scientists around the globe are testing different therapies on COVID affected patients, to find a cure for the disease, in parallel, researchers are also exploring the the therapeutic potential of existing drugs as an efficient immediate approach. From a study conducted on 1063 patients with Remdesivir, the death rate was lower as compared to placebo and the patients with more oxygen need were most benefited with this drug. This study gave a ray of hope for potential drugs for treatment, but only Remdesivir is not the drug molecule that are under clinical trials. Other several drugs can be used as a potential drug. There are 437 drug molecule structures deposited in the PubChem database (https://pubchem.ncbi.nlm.nih.gov/#query=covid-19) which are now under clinical trials. In Figure 1, we give an example of a few drug molecules that are currently undergoing clinical trials. Here we try to analyze the different chemical properties of these drug molecules to give an overview of the chemical space that all the COVID 19 drug compounds possess. During this analysis, we are highlighting the two drug molecules Remdesivir and Hydroxychloroquine that are in the news for several reasons
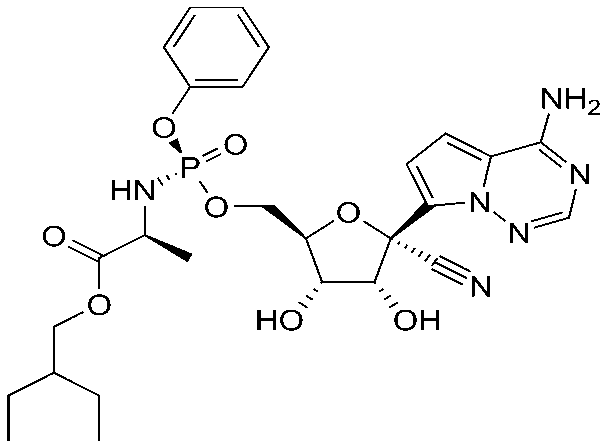
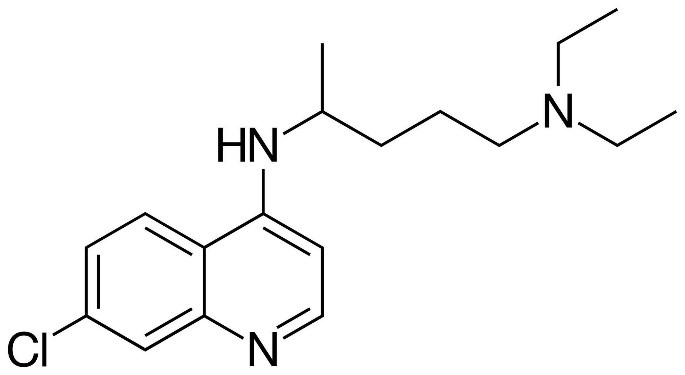
Remdesivir being a nucleoside analogue is expected to inhibit the RNA polymerase activity by terminating the RNA transcription whereas hydroxychloroquine increases the pH in endosomes and causes the inhibition of terminal glycosylation of ACE2 which prevents the entry of the virus into the host cell. These properties of Remdesivir and Hydroxychloroquine make them potential drug molecules for COVID-19. Before the drug is approved for its use, its pharmacokinetic properties have to be studied. The drug likeliness of a molecule is indicated by Lipinski's “Rule of Five (Ro5)”. It states that a molecule will be favorable pharmacokinetic properties in terms of absorption and distribution if at least two of the following criteria is met: (1) Molecular Weight (MW)≤ 500 (not too large), (2) logP ≤ 5 (not too lipophilic), (3) Hydrogen bond Acceptor (HWA)≤10, and Hydrogen bond donor (HBD). These filters help in early preclinical development and could help avoid costly late-stage preclinical and clinical failures.
| Name | Remdesivir | Hydroxychloroquine |
| Molecular weight | 602 dalton | 335.9 dalton |
| hydrogen bond donor | 5 | 2 |
| hydrogen bond acceptors | 13 | 4 |
| logP | 2.31 | 3.78 |
| Molar Refractivity | 149.83 | 98.27 |
One would think why only these two names are popping as potential drug candidates, why not other drugs. Well, we might answer that why to some extent.
A South Korean researcher has demonstrated in his studies that Hydroxychloroquine is effective in cell culture studies against Sars-CoV-2 which makes Hydroxychloroquine a good candidate as a drug by blocking replication of coronavirus causing the severe acute respiratory syndrome. The quality which makes Remdesivir unique is that it consists of a McGuigan protide which is a type of protecting group which is used to deliver as a nucleotide analog,3’ hydroxyl group of Remdesivir helps in chain termination of viral RNA, it contains a Cyano group which is a very effective inhibitor of viral RNA polymerase, the base pairing to uracil acts as a similar binding face gets incorporated into growing RNA strands by viral polymerase and Remdesivir has C-nucleoside bond which protects it from nuclease activity.
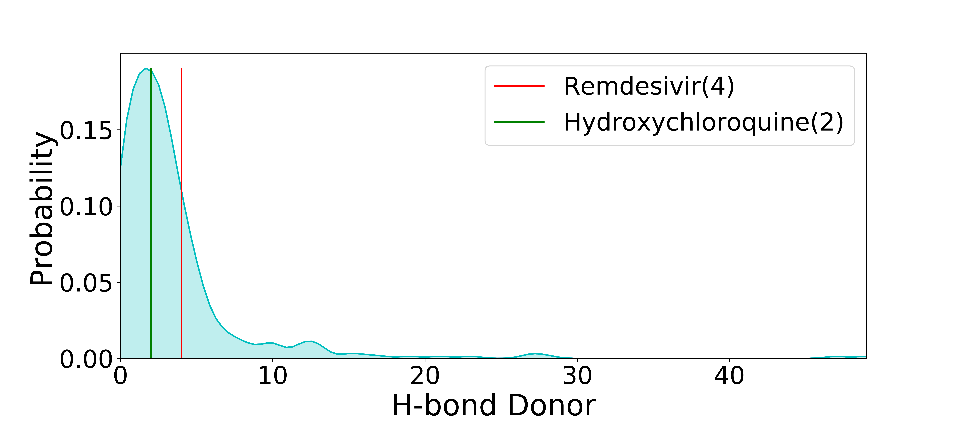
Along with the existing qualities of these drugs, a preliminary analysis is done to determine the validation of Lipinski's rule and a probability distribution has also been plotted. We analyzed the few chemical descriptors of all the 437 compounds that are currently deposited in the PubChem database. Based on Lipinski's rule of 5, the molecular weight of drug should be less than 500 daltons, as it is seen in the graph that Hydroxychloroquine has molecular weight 335 dalton which obeys Lipinski's rule and for Remdesivir, it has molecular weight 602 dalton which does not obey Lipinski's rule of 5. Polar surface area v/s Probability distribution shows Hydroxychloroquine lies in the well-distributed area whereas Remdesivir lies in the baseline of distribution. As it is known that Polar molecular surface properties predict the intestinal absorption of drugs in humans, hydroxychloroquine can be easily absorbed due to the presence of its Polar Surface Area as compared to Remdesivir. As Chiral atoms determine the activity of a drug, Chiral carbon v/s probability tells that Hydroxycholroquine lies almost at the median of the bell-shaped distribution with 1 chiral carbon whereas Remdesivir lies at the bottom with 6 chiral carbon. Heavy atoms increase the fate of survival of drugs inside the human system, the half-life of the drug is improved as it is seen in the graph there are 42 heavy atoms in Remdesivir whereas 23 in hydroxychloroquine, making them potential drug candidates with better half-life. As per Lipinski's rule, for the likeliness of drugs, it should have H-bond Acceptor more than 10. In our potential candidate; Remdesivir and Hydroxychloroquine both have H-bond donors more than 10 making them ideal drug candidates.
Though Remdesivir doesn’t follow all the Lipinski's Rule of 5 but still based on its quality it is considered as an ideal and promising candidate for treatment for COVID-19.
Besides, Ramdesivir and Hydroxycholroquine there more than 400 drug compounds are under clinical trials. Overall the chemical space of these molecules is quite broad and some of them follow the Lipisniski’s filter where some or not.
The whole world is waiting for a breakthrough either by developing new antibodies or by drug repurposing that could effectively end this global pandemic
References
- Routley, N., 2020. Every Vaccine And Treatment In Development For COVID-19, So Far . [online] Visual Capitalist. Available at: <https://www.visualcapitalist.com/every-vaccine-treatment-covid-19-so-far/>
- Oldach, L., 2020. Anatomy Of A Molecule: What Makes Remdesivir Unique? . [online] Asbmb.org. Available at: <https://www.asbmb.org/asbmb-today/science/031720/what-makes-remdesivir-a-promising-antiviral>
- In the Pipeline. 2020. Heavy Atoms, Heavy Profits? . [online] Available at: <https://blogs.sciencemag.org/pipeline/archives/2009/02/17/heavy_atoms_heavy_profits>
- Guidetopharmacology.org. 2020. Hydroxychloroquine | Ligand Page | IUPHAR/BPS Guide To PHARMACOLOGY . [online] Available at: <https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7198>
- Sciencedirect.com. 2020. Polar Surface Area - An Overview | Sciencedirect Topics . [online] Available at: <https://www.sciencedirect.com/topics/chemistry/polar-surface-area> [Accessed 15 June 2020].
- Sciencedirect.com. 2020. Polar Surface Area - An Overview | Sciencedirect Topics . [online] Available at: <https://www.sciencedirect.com/topics/medicine-and-dentistry/polar-surface-area>
- Valsler, B., 2020. Chloroquine And Hydroxychloroquine . [online] Chemistry World. Available at: <https://www.chemistryworld.com/podcasts/chloroquine-and-hydroxychloroquine/4011414.article> [Accessed 15 June 2020].
- Goodrx.com. 2020. Goodrx - Error. [online] Available at: < https://www.goodrx.com/blog/coronavirus-treatments-on-the-way/> [Accessed 15 June 2020].
- Phadke, M. and Saunik, S., 2020. COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Development Research, <https://onlinelibrary.wiley.com/doi/full/10.1002/ddr.21666>