COVID-19 is a highly contagious disease, caused by a novel coronavirus called the SARS-CoV-2, leading to respiratory illness with symptoms like dry cough and high fever. The disease has emerged in Wuhan Hubei Province, China in late December 2019 and has been declared as pandemic on March, 2020 by WHO. Till today COVID-19 pandemic is spreading at an alarming rate and has reached to 10.1 millioncases and killed 502 thousand people worldwide as of June 29,2020. As the disease spreads if an infected person coughs or sneezes near the healthy person, social distancing with infected person is very critical to stop the spread of the disease. However, for India, having a population of one hundred and thirty billion, the biggest challenge is to maintain the social distancing to prevent communities spread.
As India has a varying population density, in rural and urban areas, controlling the spread of disease is difficult. States like Maharashtra and Karnataka which has a high population density, are more prone to spread of COVID-19 compared to rural areas. The all-out-sprint in which India is making efforts to tackle the coronavirus outbreak is praiseworthy. Be it country lockdown, social-distancing or mass-surveillance of people through apps like “Aarogya Setu”, attempts are being made by the government to deal with the virus pandemic. As no vaccines and drugs are available till today, the only way to save people is to identify the affected individuals and follow social distancing with them. In this scenario rapid screening with proper test kits is prerequisite to stop the fast spreading of COVID-19.
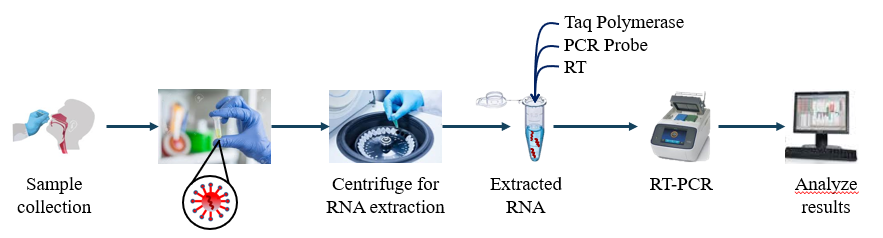
Two different diagnostic kits are currently being used to identify COVID-19 patient by the Care givers. These two kits are based on two different approaches: detection of viral RNA from throat or nasal swab specimens and detection of IgM and IgG antibodies against SARS-CoV-2 from human serum sample. The first approach, detection of viral RNA from the sample, uses real-time reverse transcription polymerase chain reaction (qRT-PCR). As SARS-CoV-2 mainly infects the lower respiratory tract of human body, samples are collected from the patient’s throat. Once the sample reaches lab, RNA extraction is done either manually through adding chemicals or centrifugation or with the use of automated kits.
Figure 1: Schematic representation of detection of COVID-19 using RT-PCR method
After extraction and purification, RNA is converted to DNA with the help of reverse transcriptase, followed by PCR amplification of the DNA as shown in Fig. 1. Additional short fragments of DNA are added to the mixture. These fragments are complementary to specific parts of the transcribed viral DNA. Presence of the virus in the sample can be detected if the fragments attach themselves to the viral DNA. Some of the genetic fragments present in the sample are used for building DNA strands during amplification, while others are used to assemble DNA and add marker labels to the strands which are then used to detect the virus. The sample is then placed in the RT-PCR machine. In the RT-PCR machine the mixture undergoes cycles, through temperatures that heat and cool the sample to activate specific chemical reactions. These chemical reactions lead to formation of new, identical copies of the target sections of viral DNA. The cycle is repeated for a number of times during which the number of the viral DNA doubles the previous number. A standard real time RT-PCR usually goes through 35 cycles. Consecutively, new copies of viral DNA are made and the marker labels are attached to the DNA strand, releasing a fluorescent dye. This is measured by the machine’s computer and displayed in real time on the screen. The computer monitors the amount of fluorescence in the sample after each cycle. When a definite level of fluorescence is transcended, this confirms the presence of virus in the sample. In order to estimate the severity of the infection, the number of cycles required to reach this level is also analysed. Few cycles denote more severity of the disease.
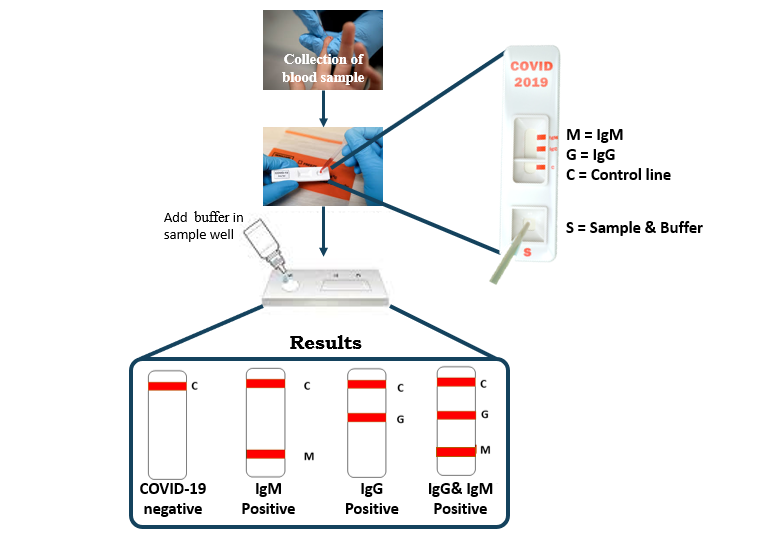
Though the RT-PCR is a standard screening technique, it has some limitations such as longer run time, only certified labs can perform the assay, requires both a suction device and a skilled operator for sample collection. In order to overcome the challenges of first test kits, another serological (blood) test kit has been developed which works on the principle of immunochromatography. As the name suggests, immunochromatography utilizes the principles of immunochemical reactions and chromatography. After a person gets infected by the virus, the immune system starts to response and produce 3 types of antibodies, IgG, IgM, IgA against SARS-CoV-2.
Figure 2: Schematic representation of Antibody testing for detection of COVID-19
These antibodies are highly specific to the recombinant antigens, containing nucleoprotein and spike protein of SARS-CoV-2, impregnated into the test strip with an indicator. As the sample blood absorbs into the porous test strip, specific antibodies (if present) from the sample blood binds to the antigen present in the test strip and wicks laterally till indicator region. An anti-human antibody, present in the indicator region of test strip, binds and holds the antigen-antibody complex in the indicator region leading to the visible colour change. The antibodies detected not only provides information about the infection but also helps us know whether there were symptoms developed from infection or the infection was asymptomatic.
Very recently, the HCG Cancer Centre in Bengaluru, has developed a new cost-effective AI-based screening approach which can detect COVID-19 with 96-97% accuracy just at a cost of Rs. 250. As, the virus mostly affects the lungs of a human body, the disease symptoms can be detected by monitoring the changes in X-ray images of lungs. The present AI-based software, introduced by HCG, has been trained on more than 10,000 X-ray data of different patients across the world including Covid-19 patients, pneumonia patients and Acute Respiratory Distress Syndrome (ARDS) cases associated with SARS COV-2. The software would process the X-rays image of a patient and predict either Covid-19 positive or not. Positive cases can be further validated by the IgG/IgM or PCR testing. It is expected that the accuracy will be 99% in near future as more X-ray images will be available.
Quantumzyme™, being an enzyme engineering company, has initiated to work in this domain. We are extending our support through our research in identifying and repurposing antibodies which will specifically target the virus and help in the treatment of COVID-19.
References
- Advice on the use of point-of-care immunodiagnostic tests for COVID-19
https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 - Iaea.org. 2020. How Is The COVID-19 Virus Detected Using Real Time RT-PCR?. Available at: https://www.iaea.org/newscenter/news/how-is-the-covid-19-virus-detected-using-real-time-rt-pcr
- Jacofsky, D., Jacofsky, E. and Jacofsky, M., 2020. Understanding Antibody Testing for COVID-19. The Journal of Arthroplasty, 35(7), pp.S74-S81.